Pharma filing strategy
Getting to grips with the patenting process is another item on the to-do list for many pharmaceutical start-ups. There are many options to consider and pros and cons to weigh-up. This is a daunting prospect to face for smaller companies who do not have the luxury of an IP department or counsel. However, there are ways to access the expertise and experience needed to use IP to reach your commercial goals.
When should I file a patent application?
Are you trying to monopolize a new chemical space early in a research program? Have you identified your lead candidate and want to get the best protection possible? Is your intellectual property an asset to attract investment or the main asset for sale in an exit strategy? Are you planning to continue with product development and marketing to generate sales and revenue for the protected asset?
All of these questions, and others, affect when it is advisable to file a patent application. The important thing to note is that the patent application needs to include data making the alleged technical effect credible and a number of worked-up examples (including synthesis routes) which justify the claimed scope. Failure to include either of these may result in a potentially weak patent application (or even an invalid patent) whilst also publicising your work and generating prior art against future patent applications. Filing too soon is to be avoided!
Of course, filing late comes with potential pitfalls too. Whilst the patent application may be better supported and more likely to stand up in litigation, there is the risk of a third-party publishing information that compromises the patentability of your work or even, more concerningly, a competitor filing a patent application which represents a potential barrier to your freedom to operate. Erring on the early side is advisable if you know others are working in the same space. If tempted to delay filing, it is also vital to have a process in place to capture potential disclosures originating internally (e.g. journal articles or clinical trial proposals), so as to ensure that an application is filed before any disclosure is made public (potentially fatal for the patent application).
Looking at filings in the pharmaceutical market which are directed to new chemical or biological entities (NCE and NBE respectively), it is very clear that timing of filing depends on the nature of the applicant.
- Start-ups and early-stage R&D streams tend to file earlier, claiming the space and seeking broad protection – this is often with a view to securing freedom-to-operate and getting granted IP early; a useful tool for attracting investment to fund further development.
- Big companies on the other hand generally file later and seek narrower protection compared to earlier more speculative filings. Filing later maximises the term of protection giving a later expiry date for the patent and related SPC.) Big companies also may have identified a lead candidate and are focussed on obtaining solid, ‘litigation-grade’ patents (plausible and inventive across the whole scope of the claims). The proposed reductions in EU data exclusivity (see here) likely render patent term even more valuable and may push innovators to delay filing to some extent.
This “file later” approach is based on the view that term of protection is key (which is unsurprising when blockbuster drugs can be worth £m per day at the end of term). Bigger companies can often negotiate to pay their way out of FTO issues (e.g. through acquisitions), and with confidence that the identified lead is the ‘best-in-class’, broad scope can be somewhat sacrificed in favour of a stronger patent case. The assumption being that the expense of bringing a drug to market is sufficiently large that competitors will not seek to develop alternatives which do not match “best-in-class” performance!
These two positions are at the extremes on the spectrum of possible filing strategies and the appropriate strategy will often evolve over time, particularly as the company grows or approaches an investment round ,for example. Best practice would involve periodic (e.g. at least annual) review of the filing approach to check it is still appropriate in the evolving circumstances.
What can I protect?
NCE and NBE filings are the most important cases in any pharmaceutical or biopharma patent portfolio, and most resource should be devoted to these. However, ancillary or secondary filings (e.g. synthetic route, formulation, combinations of APIs, polymorphs, medical use, dosage regime) can be very valuable and extend the effective term of protection for marketed products by a number of years.
It is also worth noting that if a patent application has been filed with general scope, but which does not disclose a specific compound or subset of compounds, it is often possible to protect these in a subsequent filing (even if the specific compound(s) sit entirely inside the general scope of the first case). Indeed, if one of these proves to be the lead compound, it can be essential to file a second NCE/NBE application aimed at specific APIs in order to pursue a supplementary protection certificate (SPC), for example.
Filing a subsequent NCE/NBE case which sits entirely within the claims of an earlier case is usually possible whether that is your own case, or that of a third party.
Where should I file?
The first thing to note is that there can be restrictions on where a case is filed first, depending on the nationality of the inventors, or where the invention was conceived. We can advise further on this at the appropriate time.
The global pharmaceutical market is huge (~1.48tn USD in 2022) and protection worldwide is attractive in theory, but very expensive in practice. Filing in just six jurisdictions – US, Europe, Japan, China, Canada and Brazil – covers 90% of the global market by revenue. This filing pattern can be seen even for applicants with the deepest pockets:
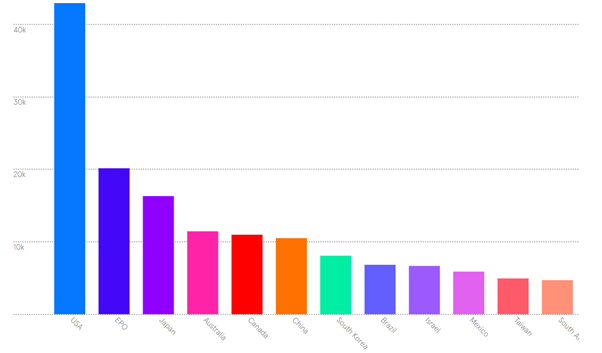
Number of patent cases by country for six of the world’s largest pharmaceutical companies.
Australia and South Korea are evidently also popular filing jurisdictions for the big multinationals. It is also worth noting that bigger companies tend to protect their blockbuster drugs with much larger filing plans; the option to do this depends on budget and on the therapeutic indications.
As with all of the issues discussed in this article, it is likely not as simple as deciding on a filing plan and adopting it for all cases. For example, NCE or NBE filings are likely worth pursuing more broadly than, for example, a formulation, polymorph or dosage regime case. Defensive filings (claiming space around your candidate but not covering the candidate per se) may have a different geographical scope again. Many bio/pharma applicants categorise cases by importance/value and each category will have a different filing list.
Moreover, it is worth considering where potential manufacturers are located. For instance, an emerging trend in publicly accessible data is for vaccine cases to be filed in India; this is a relatively small market (in sales value terms), but the expertise and manufacturing capacity of the Serum Institute means that IP protection for vaccines in India is disproportionately valuable. Israel also places quite high on the above chart, probably because of the number of generics manufacturers located there.
Finally, for a smaller company looking to collaborate with (or sell to) a bigger entity, it may be worth reviewing and/or mirroring the filing patterns of that big company to ensure that protection extends to all countries of interest. This will help make the company an attractive target.
What should I do in Europe?
Patent strategies in Europe have been completely upended by the recent opening of a new multi-state patent court and an associated multi-state patent right (the Unified Patent Court and Unitary Patent) The pros and cons of various European patent strategies are discussed at length elsewhere on this site (here); the approach taken in the pharmaceutical sphere seems broadly to be:
- Opt-out the most important cases protecting leading assets – most patentees appear to be taking their NCE/NBE cases out of the new court’s jurisdiction, at least until the UPC has been tested.
- Leave some cases in the UPC’s jurisdiction. This allows parties to shape jurisprudence and to obtain decisions in respect of a patent which are enforceable in 18 states from one case.
- For their less important filings, use the “unitary patent” as this affords relatively cheap protection across 18 states.
More generally, the biggest players seem to validate European patents in most (or all) states for their valuable cases. Smaller companies tend to be a bit more selective, with protection most commonly sought in GB, DE, FR, ES, IT, BE and NL. The first five of these states amount to ~85% of the European pharmaceutical market, and additionally covering BE and NL means protection covers most of the major ports in Europe, thereby making importation into Europe difficult for parties who might legitimately target other countries.
A further option is to request a “unitary patent”, alongside ES and GB (as these are not part of the unitary patent system). This option covers twenty European countries including the seven listed above, and at a cheaper cost (than filing 20 separate cases). There are pros and cons to this approach, and we can advise further at the appropriate time.
How to I manage costs?
We strongly recommend that a patent portfolio is reviewed regularly. If a lead compound drops away, can the geographical coverage be pruned? Or should the filing be dropped altogether? Should filings to “back-up” compounds be retained as the lead successfully passes milestones? Are the filing plans still appropriate?
The nature of pharmaceutical product development is such that the relative or perceived value of a patent family can change as the project progresses; increasing or decreasing in value depending on product performance in testing and clinical trials. A regular and centralised review process ensures that the IP portfolio is appropriate, identifies any potential gaps in the portfolio and also maximises value whilst ensuring that no rights are given up without full consideration from all areas of the business.
What other protection is available?
Many jurisdictions offer patent term extensions (under various names) for pharmaceutical products to compensate for delays in obtaining marketing authorisation. These tend to be limited to a maximum of 5 years, although this can be extended in some countries if paediatric testing is completed or if the product is an orphan drug.
Most countries also provide for periods of “data exclusivity”. During this period, generic manufacturers cannot rely on the innovator’s data when seeking marketing approval and instead would have to generate their own efficacy and safety data (which can be a significant and costly exercise). The term of data exclusivity varies around the world, but tends to be no more than 10 years (from marketing approval). The EU are currently proposing to reduce the length of data exclusivity, but with extensions available if the authorisation holder hits certain milestones (e.g. brings the product to market in all states).
Whilst data exclusivity is a useful component of the overall IP toolkit, it is a relatively weak provision. Patent term (and extensions) offer far more robust protection, making timing of the patent filing very important.

